-40%
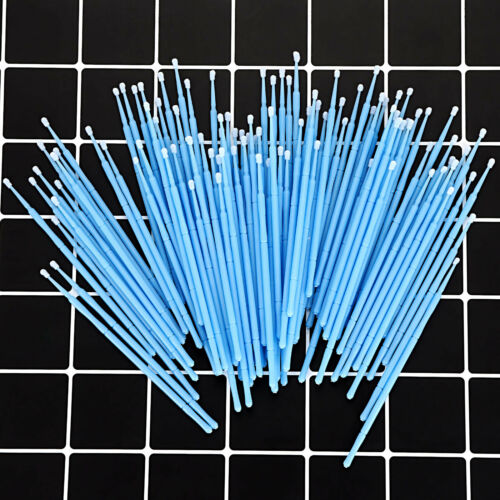
Disposable Materials Dental Eyelash Micro Brush Applicator 3 Optional Sizes
$ 3.69
- Description
- Size Guide
Description
Disposable Materials Dental Eyelash Micro Brush Applicator 3 Optional SizesMicrobrush micro applicators consist of non-linting, non-absorbent fibers arranged in a spherical shape.
It holds solutions in suspension, eliminating dripping, spilling and waste.
The bendable portion allows for precise Application in difficult to reach areas.
Disposable Micro Applicator can be used for the following:
Hemostatic solution
Disclosing solution
Sealants
Dycal applications
Etchant
Bonding Agent
Cavity Linings
Flouride Varnishing
Size:
Purple,Ultrafine
Green,Fine
Blue,Regular
Package Include:
100 Pcs/Box
The following FDA Disclaimer is required for all eBay listing in healthcare category:
The sale of this item may be subject to regulation by the U.S. Food & Drug Administration and state and local regulatory agencies.You can bid on this item if you are an authorized purchaser. If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item.If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health.
On Aug-25-21 at 12:25:10 PDT, seller added the following information: